Data
- Not currently available.
Where Does Mercury Come From?
Mercury is a naturally occurring metal from sources such as volcanoes or geothermal vents, but some areas have increased mercury levels due to human activities, including operating landfills, medical waste incinerators, municipal waste combustors, and coal-burning power plants, among others. Coal-burning power plants are responsible for 50 percent of US mercury emissions, releasing mercury into the air where it then travels long distances before depositing on land or water. Clearly mercury does not abide by political boundaries, and mercury in Rhode Island may have originated in a different state just as Rhode Island produces mercury that may not directly impact this state.
Once in water, mercury can re-enter the atmosphere, become trapped in sediment, or accumulate in fish. Different factors, which vary between different water bodies, affect which pathway to human exposure dominates. For example, waters that are more acidic (have a lower pH) tend to have fish with higher levels of mercury. By monitoring mercury levels in fish, managers can better understand which fish from which waters are safest to eat.
Bioaccumulation and Biomagnification
Mercury is usually present in water at lower levels than the more concentrated amount found in fish living in the same waters. The reason behind this intensification of amount is found in the processes of bioaccumulation and biomagnification.
Mercury contamination in fish usually begins at the very beginning of the food chain. Algae and other small organisms absorb mercury from the water. When fish eat the algae or they eat animals that ate the algae, then mercury is taken up by the fish and stored in its tissue. The fish is like a biological filter: many other compounds pass out of the fish but the mercury stays behind, accumulating in the fish over time. This process is bioaccumulation.
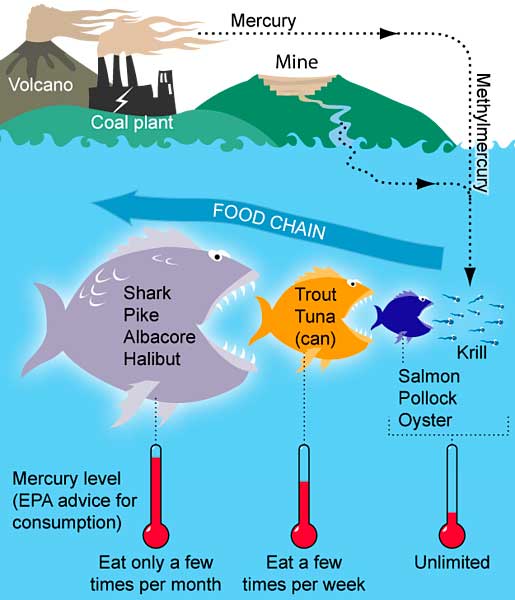
As the fish become higher and higher on the food chain (for example, a fish that eats a smaller fish that eats algae is higher on the food chain than the fish that feeds directly on the algae), the mercury becomes even more concentrated in the fish’s tissue. This is because higher up in the food chain, bigger fish eat a large number of smaller fish. So the bigger fish essentially consumes the mercury from all of the algae that each smaller fish consumed. For example, herring contains mercury levels at about 0.01 parts per million, while sharks—much higher up on the food chain—contain mercury levels greater than 1 part per million—one hundred times as high as the herring. This process is biomagnification.
One consequence of these two processes is that it can take a long time for reductions in mercury emissions to have an impact on mercury concentration in fish. Even after mercury emissions are reduced, accumulated mercury in fish tissue from previous years will continue to have an effect, especially in long-lived fish, such as striped bass.
